tags:
- chem
topic: Redox
date: 2023-10-15Introduction
key concepts
- Redox reactions involve the movement of electrons between molecules in a chemical equation.
- OILRIG (oxidation (reducing agent) is the loss of electrons, reduction (oxidising agent) is the gain of electrons)
- Basic half equations can be formed by isolating the conjugate pairs, and adding electrons as necessary (RHS: loss, LHS: gain), then balancing to equate electron amounts.
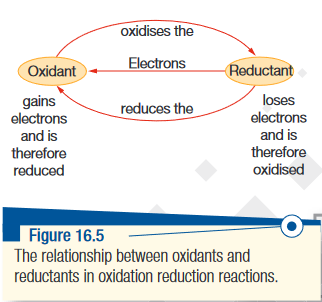
- Redox stands for reduction and oxidation.
- Refers to electron transfer in chemical reactions
- This changes the oxidation number; oxidation increases the number, and reduction decreases the number.
- ANOILRIGCAT:
- Anode - oxidation is loss of electrons - reduction is gain of electrons - cathode
- Redox reactions are essentially composed of two 'half equations', each illustrating either the gain or loss of electrons. (both are +, but reduction is on the LHS and oxidation is on the RHS).
Oxidation
- Oxidation is any of the following:
- loses electrons
- gains oxygen
- loses hydrogen
- increase in oxidation number
- Reducing agent:: The substance which undergoes oxidation in the equation, forming the 'oxidised form'.
Reducing Agents and their Conjugates
| Reducers | Oxidised Forms |
|---|---|
| sulfite ion ( |
sulfate ion ( |
| hydrogen sulfide ( |
sulfur ( |
| hydrogen peroxide ( |
oxygen ( |
| sulfur dioxide ( |
sulfate ion ( |
| bromide ion ( |
bromine ( |
| iodide ion ( |
iodine ( |
| iron (II) ion ( |
iron (III) ion ( |
Reduction
- Reduction is any of the following:
- gains electrons
- loses oxygen
- gains hydrogen
- decrease in oxidation number
- Oxidising agent:: The substance which undergoes reduction in the equation, forming the 'reduced form'.
Oxidising Agents and their Conjugates
| Oxidisers | Reduced Forms |
|---|---|
| permanganate ion (purple) ( |
manganese ion ( |
| dichromate ion (orange) (Cr2O7^2-) | chromium (III) ion (green) ( |
| hydrogen peroxide ( |
water ( |
| hypochlorite ion ( |
chloride ion ( |
| bromine (orange) ( |
bromide ion ( |
| iodine (yellow/brown) ( |
iodide ion ( |
- All of the above substances are in solution (aq), but states of matter are irrelevant.
- If the colour is not indicated next to the substance, it is colourless.
Redox Half-Equations
Full redox reactions are more complex than merely balancing conjugate pairs, typically requiring the addition of
Ion-Electron Method
- Write down 'skeleton half-equations' representing the conjugate pairs.
- Balance the number of atoms in each half equation.
- Balance the number of oxygens by adding water (
- Balance the number of hydrogens by adding
- Balance the charge by adding electrons (
- Multiply both equations to create a 'common multiple' number of electrons.
- Combine both half equations if applicable.
Examples
Simple
[multiply the entire equation by 2 to balance the electrons of the other half equation]
[RHS has charge of 4-; hence, add 4e- to LHS]
[combine half equations; electrons cancel out]
This reaction can be split into its components to symbolise what is being reduced/oxidised.
In the above equation, magnesium is undergoing oxidation and is the reducing agent whereas oxygen is undergoing reduction and is the oxidising agent.
Full
[add water in order to balance the oxygen on the LHS]
[add hydrogen ions in order to balance the hydrogen on the RHS]
[balance the charge to
This is an example of a redox half equation requiring the addition of water and hydrogen to balance each side.